MECO MPAK: Engineering a Standardized Membrane-Based Platform for PW and WFI Generation

A Technical Perspective on System Architecture, Operational Performance, and Customer Value
Pharmaceutical manufacturing depends on water systems that consistently deliver chemical purity, microbial control, and low endotoxin levels under highly regulated conditions. Among these utilities, Purified Water (PW) and Water for Injection (WFI) remain foundational, requiring both robust system architecture and long-term operational stability. Historically, distillation has served as the primary method for WFI production—a technology MECO continues to lead globally for its reliability and performance. As global pharmacopeial guidance expanded acceptance for membrane-based WFI, manufacturers gained an additional validated approach that can complement thermal systems when project conditions, utility availability, or facility strategies support a membrane-based platform.
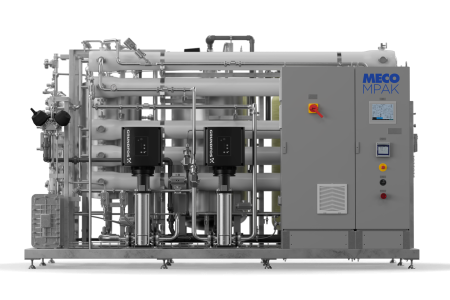
The MECO MPAK was engineered for this expanded landscape. Built on nearly a century of water purification expertise, the MECO MPAK integrates a complete packaged pretreatment system, and a membrane-based purification train into a standardized, configurable system designed to support both PW and WFI generation. The platform brings together proven purification technologies, 316 stainless steel tubing, robust automation, integrated microbial control, water sustainability and a design philosophy centered on total cost of ownership and lifecycle performance. This article provides a detailed technical examination of the MECO MPAK, exploring its system flow, engineering decisions, and suitability for modern pharmaceutical water system strategies.
A Unified System Architecture for PW and Membrane-Based WFI
The MECO MPAK is built around the principle that PW and WFI can be generated from a shared, standardized architecture when membrane science, sanitary design, and control philosophy are aligned. Rather than approaching each installation as a bespoke system, the MECO MPAK applies a consistent engineering framework—material selection, weld quality, flow geometry, drainage design, membrane configuration—and then enables site-specific adjustments through a library of configurable options including but not limited to – UV for disinfection and TOC, degassing, product heat exchanger, feedwater analyzers, and TOC analyzers. This provides the consistency pharmaceutical manufacturers seek: predictable delivery timelines, repeatable FAT/SAT execution, standardized documentation, and an operational model that translates across flow rates ranging from 1 to 100 gallons per minute.
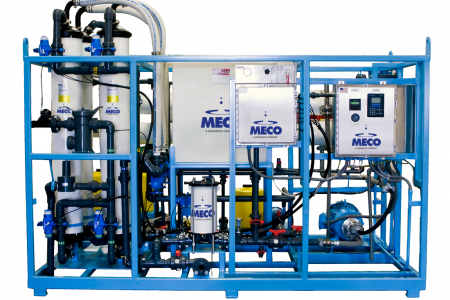
Within the system flow, pretreatment package, a 316 stainless steel break tank, reverse osmosis stages, electrodeionization (EDI), and ultrafiltration (UF) work sequentially to remove ionic impurities, reduce bioburden, and control endotoxins in alignment with PW and WFI requirements. The system’s architecture emphasizes both performance and maintainability, using a compact skid layout that provides a complete packaged and integrated system without sacrificing accessibility.
Component Quality and Material Integrity
Long-term microbial control is central to any pharmaceutical water system, and the MECO MPAK was developed with this as a foundation. The system utilizes 316 stainless steel tubing throughout all product-contact areas, executed with orbital welding to ensure weld integrity, smooth surface transitions and finish. Piping is routed for drainability, slopes are intentionally engineered, dead legs are minimized, and flow velocities are maintained at levels that discourage stagnation—all factors that contribute to controlled bioburden over time.
Every component is positioned with serviceability in mind. The clear geometry of the skid, organized valve placement, and thoughtful instrument layout reflect a design tailored not only for regulatory alignment but for routine inspection, testing, and maintenance. This foundation enables the MPAK to deliver exceptional ergonomics and reduce operating expenses for end users, while yielding consistent PW and membrane-based WFI performance across a system’s operational life.
Purification Train and Technology
The MECO MPAK’s purification sequence is designed to meet the chemical, microbial, and endotoxin limits defined for PW and WFI. The pretreatment arrangement is intended to reduce downstream equipment complications and solve the most demanding feedwater challenges. Pretreatment stages—including softening, carbon filtration, and media filtration—address hardness and organic contaminants, protecting downstream components and stabilizing RO performance. Reverse osmosis provides significant ionic reduction and initial bioburden control. The RO stage can be configured as either single-pass or double-pass, depending on feedwater quality, project requirements, and how the facility wants to balance recovery, energy use, and polishing load on downstream equipment. In a single-pass configuration, RO provides robust bulk contaminant removal for sites with well-conditioned feedwater. Where feed characteristics are more challenging or tighter conductivity margins are desired ahead of polishing, a double-pass RO configuration can be applied to enhance ionic and organic reduction.
The EDI module further reduces ionic impurities, achieving consistently low conductivity that aligns with PW and WFI requirements. Final treatment stages, including ultrafiltration (UF) with 6 kDa membranes, address endotoxin control and bioburden reduction. The MECO MPAK also offers a multitude of additional configurable options that by sequencing these technologies appropriately, and selecting the configuration to match site conditions, the MECO MPAK maintains the chemical purity and microbiological quality required for pharmaceutical use and supports compliance with global pharmacopeial expectations for PW and WFI.
Control Philosophy and Operator Interface
Automation plays a pivotal role in both performance and compliance. The MECO MPAK utilizes an industrial PLC control platform paired with a centralized Operator Interface Terminal (OIT) designed for clarity, traceability, and ease of use. The interface offers process visualization, system trending, alarm management, and secure access levels—giving operators full visibility into system state while supporting audit readiness.
Automated RO flow adjustment enables responsive control under fluctuating conditions, maintaining system stability and protecting membrane integrity. The control architecture supports defined system modes, including startup, production, standby, and defined cleaning and sanitization states, ensuring the system operates in repeatable and defensible sequences aligned with regulatory expectations.
For downstream applications requiring precise delivery, the MECO MPAK offers a pressure control package available to maintain consistent system output, providing additional operational stability within the broader pharmaceutical water system.
Microbial Control, Risk Aversion, and Sanitization Strategy
Microbial control and risk aversion are core drivers of system design, and in the MECO MPAK, CIP, and sanitization are addressed as distinct but complementary functions. The system incorporates integrated clean-in-place (CIP) capability that provides chemical cleaning coverage across the purification train, facilitating routine maintenance, and preventing fouling or biofilm accumulation.
A customer configurable sanitization schedule focuses specifically on microbial reduction. Because sanitization requirements differ between membrane-based systems and thermal distillation technologies, the MECO MPAK is designed to support heat sanitization as the primary microbial control method, but chemical sanitization is available for end user preference and system configuration. This ensures both effective microbial control and expanded capabilities that align with material and membrane limitations.
Sample points throughout the system incorporate integrated catch basins to support safe and controlled sample collection. These strategical sampling locations provide validation of effective microbial control and ensure process operations are maintained.

Sustainability and Water Recovery
As pharmaceutical companies pursue aggressive sustainability goals, water systems have become an important lever for reducing environmental impact. The MECO MPAK supports these initiatives through optimized water recovery strategies engineered into the RO and downstream stages. Depending on feedwater characteristics and system configuration, these strategies can reduce total incoming water consumption and minimize wastewater discharge without compromising PW or WFI quality.
The MECO MPAK enables energy-conscious operation, balancing pump efficiency, membrane loading, and hydraulic control to support facility-wide sustainability objectives. For manufacturers looking to scale production while shrinking their environmental footprint—or meet corporate green-initiative targets for water and energy stewardship—the MPAK provides a technically sound, resource-efficient approach to PW and WFI generation.

Facility Integration, Accessibility, and Lifecycle Performance
The MECO MPAK’s skid architecture simplifies installation by reducing field fabrication, shortening tie-in timelines, and offering a compact footprint suited for tight mechanical rooms or modular production environments. Standardized documentation also provides manufacturing consistency and streamlines FAT/SAT execution and on-site commissioning.
Maintenance accessibility is built directly into the skid layout. Key components—including the EDI module, UF assembly, valves, instruments, and sampling points—are positioned for ergonomic access and maintainability. This design supports routine service, long-term reliability, and efficient troubleshooting, all of which contribute to reduced lifecycle cost and operational downtime.
Flow rates ranging from 1 to 100 gallons per minute allow the system to be deployed across clinical, pilot-scale, and commercial manufacturing spaces without departing from the standardized architecture that gives the MECO MPAK its operational predictability.
The MECO MPAK is designed to integrate into a wide range of pharmaceutical environments—new facility builds, expansions, modular production suites, and hybrid water systems. Its membrane-based WFI capability offers flexibility for facilities where mechanical space, utility availability, or project schedules favor skid-based designs.

Conclusion
Membrane-based WFI is globally recognized and validated approach to producing pharmaceutical water, giving manufacturers a flexible pathway for adding capacity, supporting new production suites, or designing hybrid utility strategies. The MECO MPAK embodies this progression by combining standardized system architecture, 316 stainless steel sanitary construction, robust automation, integrated heat and chemical sanitization capability, and a skid layout engineered for both performance and maintainability.
For engineering teams and facility owners evaluating the role of membrane-based PW and WFI in their water system strategy, the MECO MPAK offers a technically rigorous, compliant, and operationally stable platform built on nearly a century of MECO expertise. It reflects the industry’s need for flexible, efficient, and reliable pharmaceutical water systems—and delivers a solution engineered for long-term performance in regulated manufacturing environments. Click here, to get in touch with one of our experts about the MECO MPAK today!