API Manufacturing

Pharmaceutical grade water is a critical utility in active pharmaceutical ingredient (API) manufacturing. The pharmaceutical grade water helps ensure the API manufacturing process meets regulations and produces high-quality biopharmaceutical products. Purified water that meets pharmacopeia regulations is critical to API manufacturing.
Since water is such a critical utility in many API manufacturing processes, it’s important to know about the different API process types and the grade of water that is needed for each stage of API manufacturing.
API Definition
APIs are substances that pharmaceutical companies create to use in drug products. They are intended to produce specific effects. Some drugs will incorporate several types of APIs to receive the needed treatment or action. The U.S. Food and Drug Administration (FDA) defines an API as any substance that becomes an active ingredient within a drug product or a finished dosage of a drug. These substances help with diagnosing, curing, treating, or preventing diseases.
API Manufacturing Process Types
For companies to use APIs in drug products, these substances need to go through rigorous processes that ensure their quality. A vital part of these processes is using the correct type of water for the right stages. To give a clearer idea of the required water types needed for APIs, discover the various API processes and the grades of water each uses:
1. Initial and Intermediate API
To manufacture their drug products, pharmaceutical companies will often need to put the ingredients through a chemical reaction involving multiple steps. The initial API will be the ingredient used in the first step. Besides the reaction product of the final step, the intermediate API refers to the product of each step in the chemical reaction.
In these initial and intermediate API steps, the appropriate water is municipal — local drinking water. Municipal water generally consists of standard deionized water.
2. API Cleaning
API cleaning refers to water used to clean equipment after intermediate API processes. It does not refer to the final cleaning process. In this stage, API cleaning uses water that meets local drinking water standards, such as process water.
3. API Final
The last API product manufacturing step is the final API. The final API also refers to the biologically active substance in a pharmaceutical drug. In this step, many pharmaceutical manufacturers often use United States Pharmacopeia (USP) or European Pharmacopeia (EP) Purified Water.
4. API Final Cleaning
Final API cleaning occurs after the final API. In this part of the process, the final rinse steps occur. Like with the final API, manufacturers primarily use USP or EP Purified Water.

EP/USP Purified Water Definition
The European Pharmacopeia and the United States Pharmacopeia both have strict definitions for purified water used in manufacturing APIs. Several other countries around the world also have international pharmacopeia standards for production and quality assurance requirements for pharmaceutical-grade water, including China, Japan, and India. For instance, the Chinese pharmacopeia was recently updated to quality control and technical requirements more closely aligned with international standards.
Reverse osmosis systems coupled with other filtration technologies used to produce EP and USP Purified Water remove 99.5% of constituents from feedwater sources. A robust reverse osmosis system will produce purified water that meets organic and ionic chemical purity standards and has safeguard measures in place against microbial contamination, proliferation, or recontamination.
EP/USP Purified Water Systems
With purified water being a critical ingredient in the API manufacturing industry, many companies turn to EP and USP Purified Water system experts to ensure the water used in manufacturing meets the highest quality and standards. Since purified water guidelines are notably strict, API manufacturing companies should rely on PW system experts to design, manufacture, install and service the pure water systems required for their manufacturing facilities.
MECO’s USP/EP PW Solutions
At MECO, we know how important USP and EP Purified Water production is for pharmaceutical manufacturing. As such, when our engineering team works with clients to deliver PW solutions that meet clients’ unique needs, we pay special attention to their requirements.
Our individualized approach to purified water solutions means our clients receive cost-efficient and streamlined water purification systems. These systems can accommodate easy scaling according to market growth. Our data-driven MECO smartANALYTICS™ will keep water purification systems operating at their best performance and maximum efficiency.
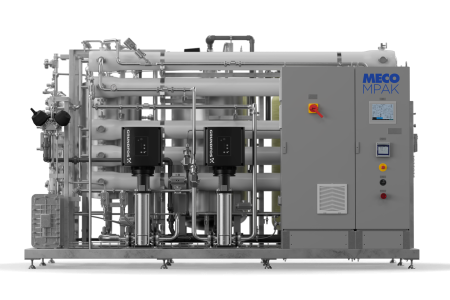
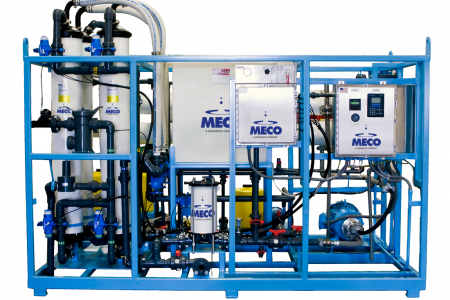
Our MASTERpak™ systems provide companies with an efficient and rigorous water purification process. We design them with the best equipment in the industry to ensure they purify water to the appropriate quality level that meets the necessary standards. Learn more about the two types of MASTERpak™ systems we offer below:
- MASTERpak™: The MASTERpak™ is a premium packaged reverse osmosis + Electrodeionization (EDI) solution. Resting on a single skid, this system produces purified water that features electrodeionization, reverse osmosis, and pretreatment for efficient purified water production. Our team can customize the system to meet a company’s specifications.
- MASTERpak™ LT: The MASTERpak™ LT is an entirely prepacked system that features an integrated control system, electrodeionization, and reverse osmosis. This “plug and play” system is easy to integrate, and customers also enjoy that the entire system sits on a single skid. With this solution in place, pharmaceutical companies receive purified water that meets or exceeds various pharmacopeia regulations while also reducing their capital costs.
Excellent service goes hand in hand with MECO’s water systems. The technologies used to produce purified water are not simple. They use sophisticated control systems to produce water to exacting standards from sources that are often variable. MECO service personnel are trained experts. When companies purchase a water system from MECO, we will deliver, install, and validate it.
After installation, pharmaceutical companies can also expect to receive responsive service to ensure their systems run at their best. With our on-site service and remote online diagnostics and monitoring, our team is prepared to help wherever and whenever a facility needs us.
Get in Touch With MECO for Purified Water Solutions
When facilities require purified water that meets international pharmacopeia standards and sustainability requirements, MECO is your trusted source. With over 90 years of water purification experience, we are a world leader in the design, manufacture, installation, and service of water purification solutions. Get in touch with one of our experts today about our MASTERpak™ product line and how we can support your facility with your Purified Water needs.