Case Study: Validated Purified Water System for CRO cGMP Manufacturing

CROs engaging in cGMP manufacturing rely heavily on substantial quantities of water. Water is essential throughout their operations, from supporting research activities to the actual production of pharmaceutical products under cGMP guidelines. Different processes within cGMP manufacturing require specific grades of water, each with stringent testing requirements and the critical need for validation.
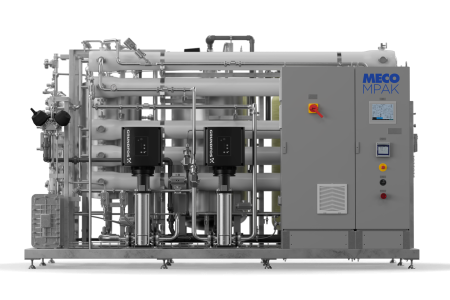
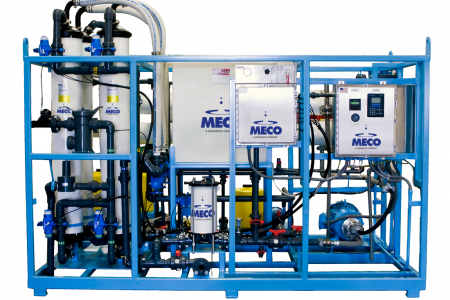
Industrial Water Purifying System Installation
The company was undergoing rapid growth and needed to relocate to a larger and more modern manufacturing facility. The organization also wanted to switch to a new, expansion-ready purification system since its existing water purification system was not movable. Water Works installed a validated Purified Water system for the company in San Diego, CA.
The Challenges
Some of the challenges the company was facing included:
- Limited scalability and future expansion: The existing water purification system was not movable or easily scalable to meet the demands of their rapid growth and relocation to a larger facility. A system that could adapt to future expansion needs was required.
- Downtime during relocation: Moving the existing water system would have caused significant downtime and disruption to manufacturing operations. The company needed a solution that ensured business continuity during the relocation process.
- High installation costs: Traditional on-site construction and installation of a new water purification system would have been expensive and time-consuming, leading to lost production time.
- cGMP compliance risks: The old water system used manual, flood-prone controllers, which posed a risk to the system’s reliability and could potentially compromise cGMP compliance for the manufacturing of commercial pharmaceutical products and clinical trial materials.
- Validation complexity: The existing system likely had complex validation requirements, making it difficult to ensure consistent water quality and compliance with regulatory standards. The CRO needed a system that simplified the validation process.
Installation Overview
Water Works designed a custom tandem skid to house the validated Purified Water system to ensure business continuity, future flexibility in moving, and reduced installation costs. The skid was designed to be able to be disconnected, put on wheels and be move-ready in 30 min. The purification system was manufactured, assembled, and tested offsite, then delivered and installed at the new location with minimal downtime in manufacturing operations.
To ensure the reliability essential for cGMP manufacturing of clinical trial materials and commercial pharmaceutical products, Water Works replaced the old manual flood-prone controllers with reliable, programmable, solid-state controllers and UL listed control panels.
Validated System Controller
Water Works selected this extremely reliable programmable solid–state system controller to ensure the system’s reliability and to simplify validation (qualification).
Why Choose Water Works for Ultrapure Water Systems in San Diego?
Here is why Water Works is the ideal choice for manufacturing water purification solutions:
- Unmatched transparency and control: We prioritize our clients’ understanding and control over their system by using non-proprietary materials. Comprehensive documentation detailing maintenance procedures, material origins, and straightforward reordering processes back this commitment. It also makes it easier to comply with stringent industry regulations such as EUDRALEX, FDA, and ICH guidelines.
- Streamlined production and validation: We significantly reduce production timelines and validation expenses. Our in-house Factory Acceptance Testing (FAT), where we complete 98% of the work at our facility, minimizes disruptions at a site and accelerates project completion. We can also accommodate expedited delivery requests.
- Comprehensive life cycle support: Beyond installation, we offer a complete suite of services to guarantee the long-term optimal performance of our system. This suite includes flexible maintenance and validation contracts. While we provide 24/7 emergency support, our primary focus is on predictive maintenance to ensure continuous, efficient operation.
Partner With MECO for Quality Validated Purified Water Systems
Water Works broadens MECO’s range of products to include high-purity water systems with smaller capacities, complemented by outstanding service capabilities across California. With a decades-long legacy, MECO has consistently led the way in water purification technology, providing efficient and environmentally conscious solutions. Water Works offers specialized knowledge and dedication to delivering innovative Ultrapure Water systems for the life sciences and high-technology sectors, complementing MECO’s established expertise.
Contact us today for more information about our water purification technologies.