Water Quality Standards Guide

Water plays a central role in dozens of industries. Pharmaceuticals, biotechnology, and life sciences depend on water purity to produce consistent results and uncontaminated products. Without adherence to water standards, an operation risks its compliance, reputation, equipment, and consumer health.
High water purity is critical for keeping a facility productive and successful. Understanding how standards influence water quality and how facilities can achieve this more effectively allows operations to improve efficiency while reducing risk.
In this guide, we provide comparison charts, water quality conversion charts, USP and ASTM water quality standard parameters. You can also download the charts used in this guide below.
Download Water Quality Standards Guide
Why Water Quality Regulations Exist
Water quality standards protect public health. Water contaminants are common and often undetectable without specialized equipment. If chemicals, bacteria, or radionuclides contaminate a facility’s water, it can lead to adverse health effects or poor product quality. An industry may use water as a cleaning agent or raw material. Any contamination will directly affect the end result.
Regulatory agencies like the United States Pharmacopeia (USP) establish water guidelines for each application. These standards govern limits for inorganic and organic compounds, microbial growth, conductivity, and Total Organic Carbon (TOC). With clear limits for each industry, a facility can protect batch consistency and equipment function. Leaving contaminants in water introduces risky variables that affect outcomes and productivity.
Beyond compliance, water purity standards protect science. If manufacturers can maintain their water purity, they can produce consistent products that work effectively without compromising human health. Set standards establish industry consistency, giving facilities clear guidelines to follow and audit.

How Water Quality Standards Affect Industries
Ultrapure Water is critical for the manufacturing and research sectors. Minuscule contamination can lead to compromised results or unwanted microbial growth. Many industries rely on water quality standards to protect their work, including:
- Medical devices: This industry uses water for rinsing, lubrication, and surface preparation. Particulates, microbes, and organic residue interfere with sterilization and compromise device integrity.
- Pharmaceuticals: Pharmaceuticals and biopharmaceuticals rely on water purity to maintain product safety and efficacy. Contaminated water impacts drug formulations and sterile injectables. Particles and microbes change product chemistry, affecting quality and patient health.
- Chemical processing: The chemical processing industry depends on consistent water composition to deliver predictable yields, maintain regulatory compliance, and protect sensitive equipment.
- Semiconductors: Microelectronics and semiconductors use Ultrapure Water for rinsing wafers and photolithography. Contaminants will affect performance and result in defects.
- Food and beverage: This industry must monitor process water for chemicals and microbes to preserve product taste and stability.
Key Water Quality Standards for Life Sciences and Healthcare
An operation’s relevant water quality standards depend on its country, industry, and application. Standards govern contamination levels based on where and how water is used. Understanding the major quality guidelines can help facilities adhere to the relevant regulations.
Life sciences and healthcare have the following water quality standards:
- USP (United States Pharmacopeia)
- AAMI ST108:2023 (Association for the Advancement of Medical Instrumentation)
- Clinical Laboratory Reagent Water (CLRW)
- ASTM (American Society for Testing and Materials)
Below is a summary table of all these key standards. Each standard is broken out and information is provided on the purpose and use below the table. Many of these standards overlap and can be somewhat interchangeable dependent on the application.
Realistically, the best water quality standard is one designed by the end-user and considers the specific application and critical parameters, and the end user should be consulted when determining the targets.
Producing water to each standard is often done with equipment that is specifically designed to meet the quality requirements. In many cases, multiple water qualities are needed, and it is common to design a central system that can meet the lower quality requirements and polish to the higher quality where it is required. MECO can help design a system to meet standards and optimize for efficiency, sustainability, and total cost of operations.
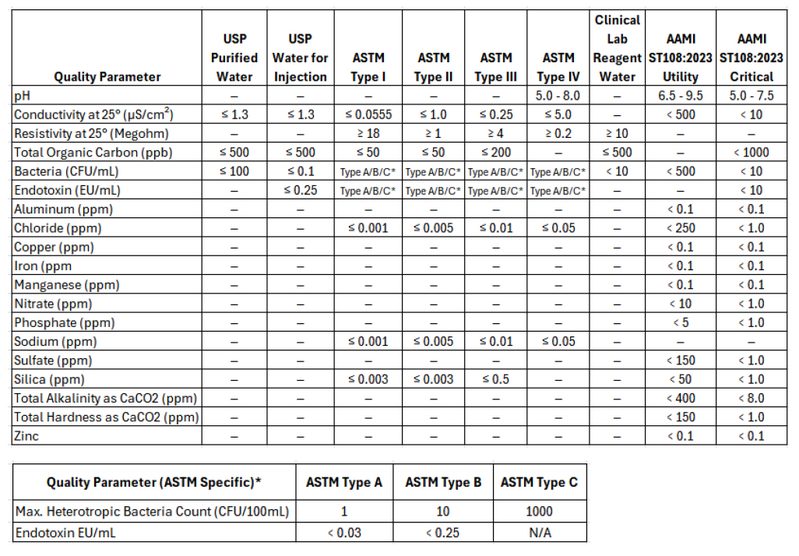
USP Purified Water
The USP’s purified water quality standards regulate pharmaceutical and biopharmaceutical manufacturing water. These standards focus on Purified Water and Water for Injection (WFI), establishing the baseline for water in controlled facilities.
Purpose and Use for Purified Water: USP Purified Water is intended for use in the preparation of non-parenteral pharmaceuticals and is used in various pharmaceutical processes such as cleaning equipment and preparing bulk chemicals. It is one of the several types of water defined by the United States Pharmacopeia (USP) to ensure the quality and safety of pharmaceutical products.
USP Water for Injection (WFI)
Purpose and Use for WFI: USP Water for Injection (WFI) is a high-purity water that is primarily used in the pharmaceutical and biotechnology industries to produce parenteral (injectable) drugs. It is also used in the preparation of solutions for intravenous administration, as well as in the cleaning and rinsing of equipment and containers that come into contact with injectable products.
Production of WFI is typically produced by distillation or reverse osmosis followed by ultrafiltration, to ensure the highest levels of purity. The production process must be validated to consistently produce water that meets all the required specifications.
| Quality Parameter | USP Purified Water (PW) | USP Water for Injection (WFI) |
|---|---|---|
| Conductivity at 25° (μS/cm²) | ≤ 1.3 | ≤ 1.3 |
| Total Organic Carbon (ppb) | ≤ 500 | ≤ 500 |
| Bacteria (CFU/mL) | ≤ 100 | ≤ 0.1 |
| Endotoxin (EU/mL) | – | ≤ 0.25 |
Based on the water type, facilities must meet limits in these areas:
- TOC: Organic compounds may interfere with downstream production or support microbial growth. TOC must be under or equal to 500 parts per billion (ppb).
- Conductivity: This metric quantifies the ionic contamination in water. Purified Water and WFI should be at or equal to 1.3 microsiemens per centimeter (μS/cm).
- Bacteria: Bacteria count assesses water for microbes that could multiply and impact health or product performance. Purified Water’s bacteria count must be less than or equal to 100 colony forming units per milliliter (CFU/mL), while WFI should be less than or equal to 0.1 CFU/mL.
- Endotoxins: Endotoxins can lead to negative immune responses, such as fever, when consumed. Endotoxin count is only measured in WFI. The count should be at or under 0.25 endotoxin units per milliliter (EU/mL).
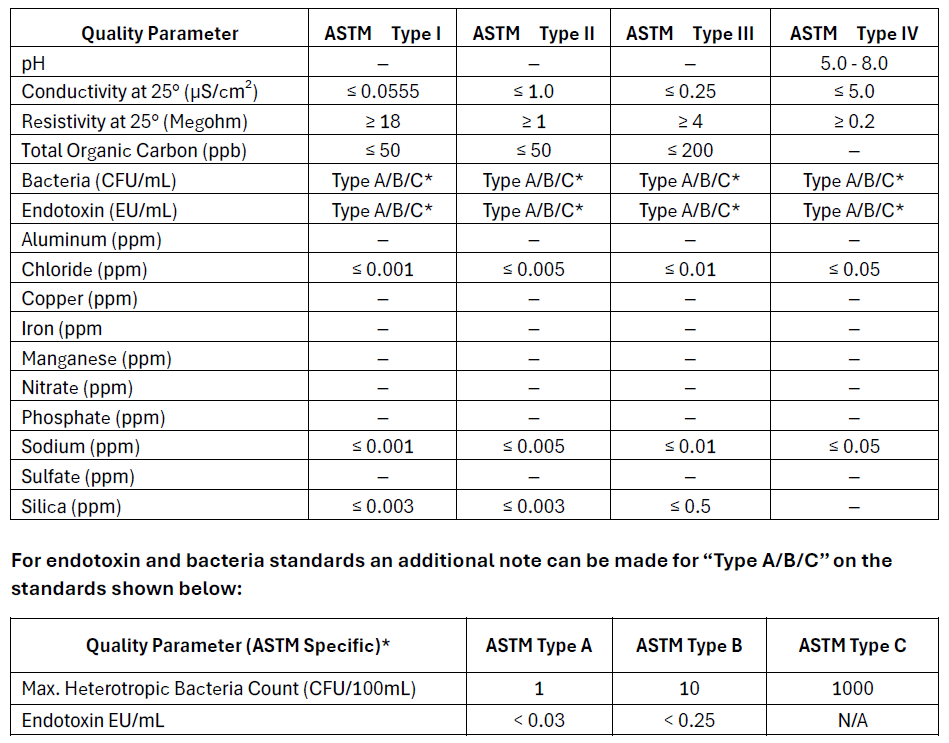
ASTM (American Society for Testing and Materials)
ASTM standards are often referenced in industrial, environmental, and research settings where specific purity levels are needed but may not necessarily align with pharmaceutical-grade requirements. USP standards are primarily used for pharmaceutical and medical applications where sterility and absence of pyrogens are critical. ASTM standards provide a broad framework for water purity used in a variety of non-pharmaceutical settings, while USP standards are focused on ensuring the safety and efficacy of water used in medical and pharmaceutical contexts. However, because they have much overlap, the USP standards are more frequently used as a target over the ASTM standards. Most modern central ultrapure water systems aim to meet USP Purified Water Standards. The USP specifications exceed ASTM Type II water specifications. In the case where a point of use requires ASTM Type I water, a desktop polisher is commonly used to polish the water to meet this standard.
- Type I: Analytical procedures requiring minimal contamination, ultrapure water for critical applications (e.g., HPLC, ICP-MS).
- Type II: H Laboratory experiments and general analyses high-purity water for general lab use (e.g., reagent preparation).
- Type III: Preliminary cleaning processes. RO water for basic tasks (e.g., glassware washing).
- Type IV: General laboratory water for non-critical tasks (e.g., rinsing glassware).
Clinical Laboratory Reagent Water (CLRW)
Purpose and Use for Reagent Water: Clinical Laboratory Reagent Water (CLRW) is a high-purity water specifically designed for use in clinical laboratories. It is used in various diagnostic and analytical procedures to ensure accurate and reliable test results. The water quality standards for CLRW are defined by the Clinical and Laboratory Standards Institute (CLSI).
| Quality Parameter | Clinical Lab Reagent Water |
|---|---|
| Resistivity at 25° (Megohm) | ≥ 10 |
| Total Organic Carbon (ppb) | ≤ 500 |
| Bacteria (CFU/mL) | < 10 |
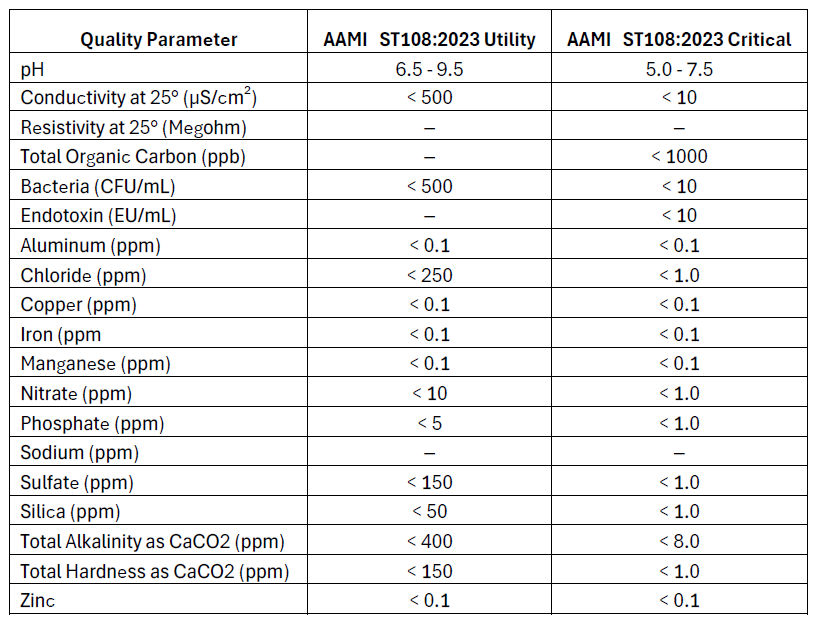
AAMI ST108:2023 (Association for the Advancement of Medical Instrumentation)
Purpose and Use: This standard primarily applies to healthcare facilities using water in the sterilization of medical devices. The primary goal of AAMI ST108 is to ensure that water used in cleaning, disinfecting, and sterilizing medical devices meets specific quality criteria to prevent contamination and ensure patient safety. The standard emphasizes the importance of water quality in all stages of medical device processing, from initial washing to final rinsing and steam sterilization. This standard is commonly referenced in hospital sterile process departments (SPD) where sterilization equipment will have two connections, one for critical water, and one for utility water.
- Utility Water: In initial cleaning stages, such as washing, soaking, or flushing, facilities can use Utility Water. It has less strict requirements for conductivity, bacteria, and various minerals. The goal of Utility Water is to prevent instrument damage or issues with cleaning.
- Critical Water: Final rinses and sterile processing areas rely on Critical Water to ensure maximum purity. Critical Water must meet tighter restrictions for microbial content, minerals, TOC, and conductivity. Otherwise, water may contain contaminants, affecting patient safety and sterilization.
Learn more about Pharmacopeia guidelines for Pure Water here.
The Effects of Noncompliant Water Use
Failing to meet the above standards goes beyond affecting end performance. It is also a risk to any business. Contaminated water compromises product integrity, skews results, and increases health risks. For example, poorly sterilized medical devices can injure patients. Elevated microbial counts may lead to health concerns.
Groups like the Food and Drug Administration (FDA) monitor health and food products. If a facility violates water quality standards, it risks audits and fines. Noncompliant facilities may have to throw out product batches, shut down production lines, revise procedures, and pay fines. These interruptions are costly and time-consuming.
Facilities also risk their reputation with noncompliant water quality. A single contamination event can have significant effects on a facility’s reputation. Regulators and customers could turn to competitors long-term. Frequent audits after a violation slow down production, affecting an operation’s ability to meet demand. Businesses must invest in high-quality water systems and procedures to limit contamination.
How Facilities Can Meet Water Quality Standards
With multiple life sciences water quality standards for different applications, facilities may struggle to balance various filtration solutions. A pharmaceutical manufacturer may need WFI for product rinsing, CLRW for clinical diagnosis, and Purified Water for formulation.
There are various methods used to meet different water purification goals. Some purification solutions feature multiple methods to ensure a higher purity level. Understanding each method helps facilities find a solution that meets their needs.

Reverse Osmosis
Reverse osmosis uses membranes to purify water. The membrane removes microbes, organic compounds, and dissolved ions. The result is water that has completed the first stage toward high purity designation. Use reverse osmosis treatments to achieve Purified Water or ASTM Type II.
It is common for these systems to feature pretreatment softening or filtration steps to extend the membrane’s life. Combine reverse osmosis with further purification measures like deionization to improve purity.
Vapor Compression Distillation
Vapor compression distillation evaporates water and compresses the resulting steam to produce WFI. The high temperatures and closed-loop design make this solution highly effective at eliminating microbes. Choose vapor compression systems for energy efficiency and effective purification.
Multiple Effect Distillation
Multiple effect distillation (MED) also generates WFI. The system sends water through several evaporators, delivering an energy-efficient result. The steam from each evaporator powers the next one. MED systems operate at higher temperatures than vapor compressors. However, they need a reverse osmosis feature to achieve compliant WFI. Choose MED systems for smaller WFI output.
These systems create the Pure Steam needed for the life sciences and pharmaceutical industries. The steam involved in these processes must be Purified Water to prevent contamination. An evaporator heats Purified Water, turning it into steam that passes through a separator to become dry and contamination-free. Facilities can use Pure Steam to sterilize equipment or humidify areas without the risk of corrosion or microbes.
Maintaining Compliance Through Monitoring, Maintenance, and Automation
Meeting water quality standards goes beyond system installation. Once a facility has effective water treatment systems, it must ensure they remain effective. Ongoing compliance means active monitoring and regular inspections. If water quality falls, an operation must be ready to respond.

Continuous Monitoring
Purification systems work to reduce critical parameters like TOC and bacteria, but businesses cannot rely on these systems alone. Best practices include equipping systems with measures for sample collection and monitoring to prevent water contamination.
MECO’s smartANALYTICS™ platform provides real-time visibility into system performance. Users can track water contamination and use automated alerts or trending tools to find patterns and address quality concerns. With this technology, facilities can maintain constant compliance while spotting gradual performance concerns and identifying TOC changes or WFI membrane fouling before they compromise water quality.
Maintenance
All systems need regular maintenance to protect performance. MECO builds systems with calibration and validation in mind, offering solutions with accessible features and detailed documentation packages. These offerings keep facilities ready for Good Manufacturing Practice (GMP) audits. The better a business maintains its systems and documents that care, the easier it is to remain compliant.
Automation
Automation is a critical tool for maintaining compliance. Water purification systems with automation services provide facilities with greater peace of mind. Instead of relying on manual documentation and sanitization, purification systems can streamline the work. MECO’s Ultrapure Water System called MASTERpak™ MICRO comes with integrated control system. They log critical data and automate sanitization cycles for more efficient compliance monitoring.
Meet Water Quality Standards With MECO
In highly regulated environments, water quality must meet a range of application-based demands. Partner with MECO and get support from a company that works directly with experts to deliver long-term water solutions. Our engineers collaborate with facility teams to define the exact purity levels needed for each application.
After setup, the MECO MASTERsupport™ Service Center helps facilities stay efficient and compliant. We provide remote monitoring, operator training, and life cycle support for long-lasting water purification. We have helped the world’s most advanced facilities meet rigorous standards for over 90 years.
Reach out to us online to learn how we help operations achieve their water purification goals.