Case Study: Advanced Water Purification Solutions for Biopharmaceutical Manufacturing
High-purity water systems are essential to maintaining quality and reliability in pharmaceutical and medical device manufacturing. MECO’s experience in delivering engineered solutions for cardiac and vascular care product facilities underscores our commitment to supporting industries where precision, compliance, and innovation are critical.
By integrating advanced technologies such as reverse osmosis (RO) membranes and high-purity materials, MECO delivers tailored purification systems designed to meet the most demanding regulatory standards.
The Role of Molecular Biology-Grade Water in Lab Applications
Biopharmaceutical manufacturing often relies on molecular biology-grade water to enable accurate, repeatable scientific outcomes. Processes such as genetic testing, aseptic manufacturing, and cell culture require a water supply that is consistently free of microbial and organic contaminants.
MECO supports these needs through purpose-built purification skids, robust instrumentation, and integrated control systems—ensuring product integrity and compliance throughout R&D and production.
Leveraging RO Membranes and System Efficiency
High-efficiency reverse osmosis membranes are a cornerstone of modern purification. They remove soluble impurities that can interfere with production or affect research accuracy. MECO’s systems utilize ultra-low-pressure RO membranes to enhance energy efficiency while delivering high-performance results.
Project Profile: Purified Water System for a Healthcare Products Facility – Temecula, CA
MECO installed a validated purified water system for a major GMP manufacturing facility in Temecula, California. This global medical device company specializes in cardiac and vascular care solutions.
Project Background
The customer required a purified water system to support new QC laboratory operations as part of an expanded facility footprint.
Unique Challenges
The project demanded rapid execution on an aggressive timeline, while meeting the high standards of GMP compliance and operating within the constraints of a sensitive and space-limited site.
Project Outcome
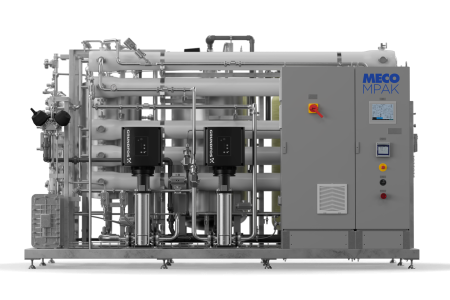
To ensure consistent performance, easy maintenance, and control essential for GMP manufacturing, we installed our most popular system, the ES-1 (known now as the MASTERpak™ MICRO), to produce validated low organic and microbial contaminants 18 Megohm water. This validated system was constructed with high-purity materials using infrared-fused PVDF pipes, valves, and fittings.
Key features included:
- Up to 50% energy savings through ultra-low-pressure RO membranes and high-efficiency components
- Compact design to accommodate a mezzanine-level installation
- UL-listed solid-state system controller for safe operation above GMP production spaces
- Comprehensive documentation package to streamline commissioning and validation
- Accelerated project timeline: completed in just 5 weeks, reducing typical lead time by half
To successfully limit the footprint, we designed a custom, small-footprint system that fit on the mezzanine located on the top floor, above the labs and manufacturing area. To ensure flood protection of the equipment and operations on the lower levels of the buildings, the Water Works team built the system to be operated by a UL-listed, solid–state system controller.
Finally, our team delivered a comprehensive documentation package to provide commissioning support for quick and easy validation. Per the customer’s request, our team used an accelerated project schedule, essentially cutting the lead time by 50% and completing it in 5 weeks.
Let’s Build Your Next Breakthrough—Together
MECO’s integrated water purification solutions combine electrodeionization (EDI) water treatment and reverse osmosis technologies to comprehensively support lab quality control and manufacturing operations. Our systems deliver molecular biology-grade water that meets the highest purity standards, ensuring the reliability and accuracy of biopharmaceutical products.
By using advanced RO membranes and high-purity materials, MECO ensures that water purification systems are efficient and sustainable, offering significant energy savings and reduced operational costs. Our expertise in designing custom, small-footprint systems allows us to meet the unique spatial and operational requirements of organizations like the Cardiac and Vascular Care Products Manufacturing Facility in Temecula, California.
MECO’s water purification systems are engineered to support high-purity, high-performance environments across the life sciences. Whether for laboratory QC, R&D, or full-scale GMP manufacturing, we provide validated, sustainable solutions that meet the highest standards for reliability, efficiency, and compliance.
Contact MECO to discuss how we can support your next breakthrough.