Case Study: Validated Purified Water System for a Diabetes Device Manufacturer
Diabetes device manufacturing demands high-purity water that meets United States Pharmacopeia (USP) standards for Purified Water and Water for Injection (WFI). These standards are critical to ensuring the safety, perf

ormance, and regulatory compliance of finished medical products. Validated Purified Water systems are engineered to deliver consistently compliant water quality across every production cycle. At MECO and Water Works, we specialize in delivering engineered water systems that meet the rigorous demands of pharmaceutical and medical device manufacturing.
Why Trust Water Works Inc. for Validated Purified Water Systems?
Water Works Inc., now part of MECO and Grundfos, brings decades of experience in the design, fabrication, and installation of high-performance water systems for regulated industries. With a dedicated team in California, Water Works offers tailored solutions that reflect both the local environmental conditions—such as drought resilience—and the stringent standards of life sciences manufacturing.
As part of MECO, a global leader in Water for Injection and ultrapure water technologies, Water Works now has access to an expanded portfolio of solutions. These include UV-TOC reduction, ultrafiltration, and electrodeionization (EDI), enabling a comprehensive approach to high-purity water systems for regulated environments.
The Importance of a Validated Purified Water System for Diabetes Equipment
Validated Purified Water systems are essential to the manufacture of diabetes equipment, where water is used in cleaning, rinsing, and sterilization processes.
Any impurities can compromise product sterility, affect device accuracy, or introduce risk to patient safety.
To mitigate these risks, systems undergo rigorous validation protocols—Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—to align with current Good Manufacturing Practices (cGMP). These validations confirm that the system operates within defined specifications and delivers consistent, compliant water quality.
Project Overview: San Diego, CA
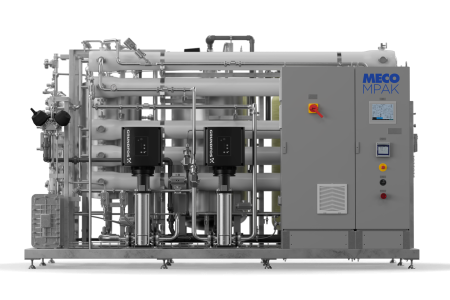
Water Works, Inc. completed the installation of a validated Purified Water system for a continuous glucose monitoring (CGM) system manufacturer in San Diego, CA.
The client needed to duplicate their existing Purified Water system to support a newly opened manufacturing line. The existing system was meeting their needs, but their expansion demanded a second, identical system to maintain production capacity and quality standards.
Our Solution
The Water Works team leveraged its proven ES1 system architecture, incorporating targeted enhancements to support advanced Process Analytical Technology (PAT). Upgrades included:
- PLC-based control logic for streamlined operation
- Human-Machine Interface (HMI) for intuitive system access
- Integrated online Total Organic Carbon (TOC) monitoring
These features enabled real-time water quality tracking and rapid response to system deviations—supporting proactive compliance and process optimization.
TOC monitoring, in particular, plays a critical role in detecting organic contaminants and aligns with USP <643> requirements. It serves as a key performance metric and an early warning system for microbial risk.
The Result
The upgraded system achieved a 10-fold (1-log) reduction in TOC levels, exceeding the manufacturer’s internal water quality specifications. The design also reduced the system’s physical footprint by 30%, improving space efficiency and flexibility for future expansion.
The result: a robust, validated solution that strengthens production resilience, reduces risk of contamination, and ensures consistent compliance with regulatory standards.

MECO Water Systems for Medical Device Manufacturing
MECO delivers sustainable, validated water purification systems for the medical device industry, supported by comprehensive services that include:
- Reverse osmosis and EDI
- Distillation and pure steam generation
- TOC reduction and microbial control
- Storage and distribution
- DI tank exchange and preventative maintenance
Our systems are engineered for reliability, precision, and compliance—and backed by a century of expertise in high-purity water.
Let’s build your next breakthrough—together. Contact us to discuss a water purification system designed for your manufacturing needs.