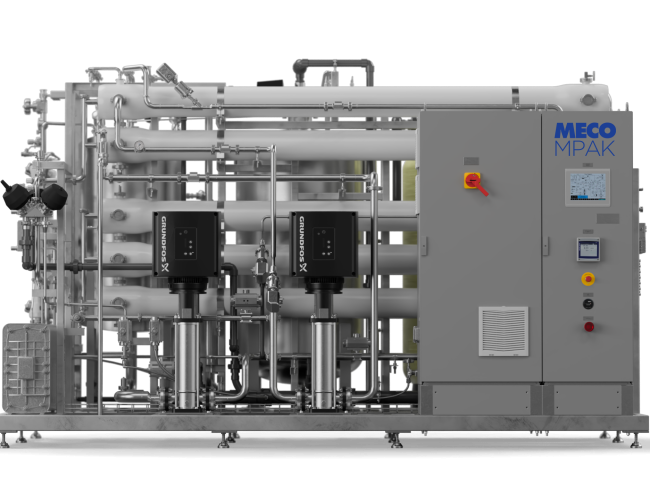
Built on nearly a century of water purification expertise, the MECO MPAK combines expert engineering with next-generation membrane capability. Designed for precision, reliability, and compliance with all global pharmacopeia, MECO MPAK delivers PW and WFI-grade water in one, packaged configurable system. MECO MPAK is the #1 solution in membrane purified water (PW) and water for injection (WFI) generation and testing.
Our membrane-based WFI turnkey system’s design minimizes footprint and installation time. The system features optimized water recovery and energy savings to meet the highest sustainability targets. Best-in-class components ensure purity and durability throughout the system. An intuitive Operator Interface Terminal provides easy monitoring and navigatable system control. Accessible sample points with integrated catch basins offer safety and ease of use. An integrated break tank maintains system balance and enables convenient tuning.
This pharmaceutical reverse osmosis complete system is engineered and manufactured in accordance with current ASME BPE standards and ISPE guidelines. It meets all global pharmacopeia requirements for both Purified Water and ambient Water for Injection. The MECO MPAK WFI system is fully compliant with FDA, cGMP, and GAMP requirements.
For more information and cost for our membrane WFI systems, please contact our team.
| MODEL | UNIT | FLOW RATE |
|---|---|---|
| MPAK1D | GPM (m3/h) | 0.9 – 2.6 (0.2 – 0.6) |
| MPAK2D | GPM (m3/h) | 2.3 – 5.7 (0.5 – 1.3) |
| MPAK3D | GPM (m3/h) | 4.1 – 9 (0.9 – 2) |
| MPAK4D | GPM (m3/h) | 5.7 – 13.5 (1.3 – 3.1) |
| MPAK5D | GPM (m3/h) | 11 – 27 (2.5 – 6.1) |
| MPAK6D | GPM (m3/h) | 25 – 52.5 (5.7 – 11.9) |
| MPAK7D | GPM (m3/h) | 35 – 81 (7.9 – 18.4) |
| MPAK8D | GPM (m3/h) | 50 – 110 (11.4 – 25) |
The next generation of Membrane-based pharmaceutical-grade water.