Water for Injection Generation – A Critical Utility
Pharmaceutical-grade water is key in the life science industry. Due to its use in the pharmaceutical and medical fields, this water is heavily regulated by Pharmacopeia regulations to ensure patient safety. However, with the multiple purification systems on the market, finding the best production method can be difficult. This piece will dive into the different types, uses, and methods of purification available for Water for Injection.
What is Water for Injection (WFI)?
Water for Injection, commonly referred to as WFI, is a solvent used to dilute medications or solutions that will be injected into the body. It is a pharmaceutical vehicle that delivers drugs intravenously (parenteral preparations). Other applications include using it as a fluid replenisher, in inhalation medications, and in some ophthalmic (eye health) products. By regulation, it cannot contain any other substances, including agents that inhibit bacterial or microorganism growth.
Water for Injection (WFI) distillation is primarily achieved through three different manufacturing processes: multiple effect distillation, vapor compression distillation and membrane-based systems. Usually, WFI systems utilize high-temperature (above 80° Celsius) environments, and continuously-circulating water systems. This elevated temperature method prevents microbial growth in the water.
Comparison of Water for Injection and Purified Water
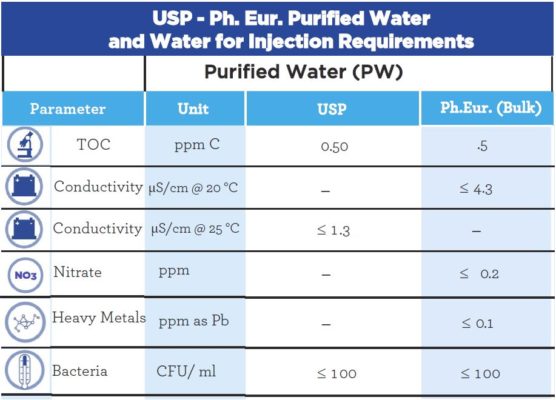
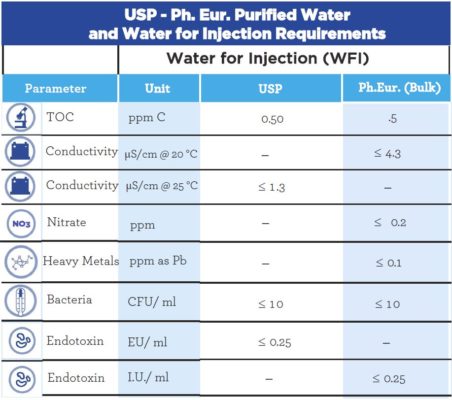
The difference between Water for Injection (WFI) and Purified Water (PW) lies in their physical, chemical, and microbiological properties. Pharmacopeias determine the properties used to define these products, and a comparison of these requirements for the U.S. Pharmacopeia and European Pharmacopeia is represented in the tables below.
Purified Water systems typically include two stages that treat the feed water. Pretreatment is the initial stage and modifies the water quality to reduce or eliminate any substances that can be harmful to downstream unit operations. These substances include suspended solids, hardness, and disinfectants (chlorine/chloramines). The second stage is the final treatment, where pretreated water enters reverse osmosis membranes before passing through an Electrodeionization (EDI) module or other deionization process. Deionization further polishes the water to lower its conductivity.
It is important to note that not all systems have the reverse osmosis (RO) stage. The RO membrane is mostly found in modern purification systems. Additionally, some high-performance Purified Water systems include distillation. Vapor Compression distillation is the most widely used method because it can generate ambient water without requiring an external cooling medium. Purified Water can be used in non-parenteral preparation for pharmacology, such as tests and analyses.
However, there are only three acceptable methods for producing pharmaceutical Water for Injection. Approved methods include:
- Multiple-effect distillation
- Vapor compression distillation
- Membrane-based reverse osmosis
WFI systems usually utilize high-temperature (above 80° Celsius) environments and continuously circulating water systems. This elevated temperature method prevents microbial growth in the water.
Types of Water for Injection
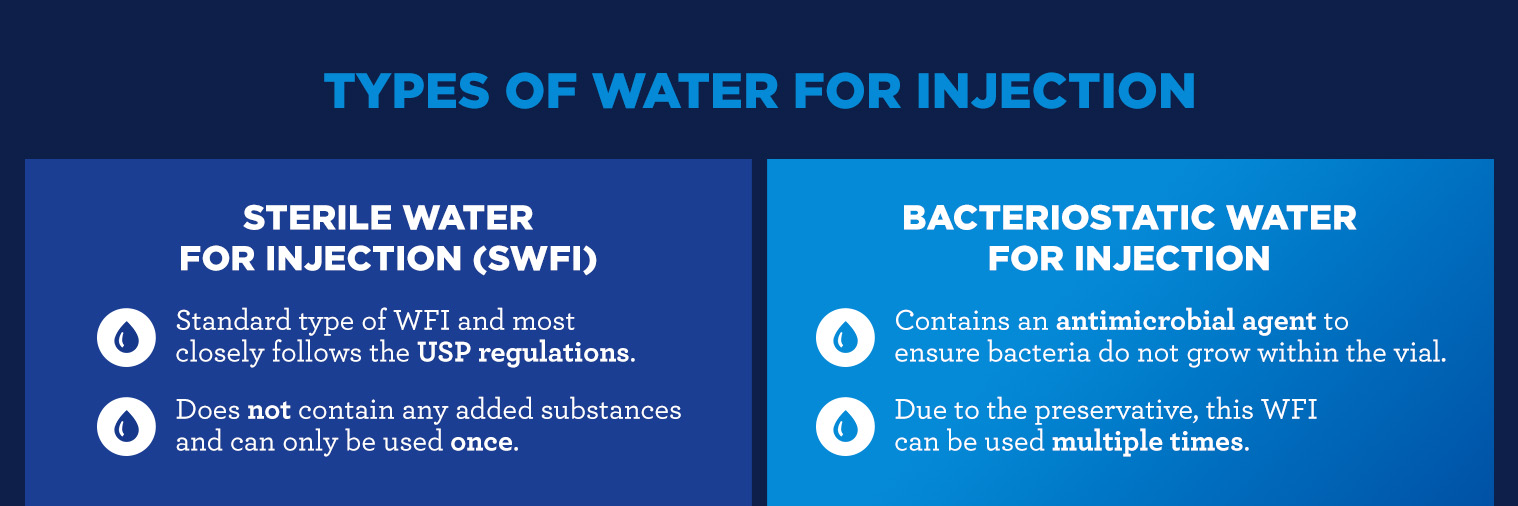
There are two main types of Water for Injection:
- Sterile Water for Injection (SWFI): This is the standard type of WFI and most closely follows the USP regulations. It does not contain any added substances and can only be used once.
- Bacteriostatic Water for Injection: Bacteriostatic water is manufactured with an additive. It contains an antimicrobial agent to ensure bacteria do not grow within the vial. The antimicrobial agent is the main difference between the bacteriostatic and sterile Water for Injection. Due to the preservative, this WFI can be used multiple times.
Uses of Sterile Water for Injection
Sterile Water for Injection has multiple uses in the medical and pharmaceutical fields, such as:
- Diluting medicine: One of the primary use cases of SWFI is to dilute medicine administered through intravenous injections.
- Rehydrating lyophilized powders: WFI is used to reconstitute medicine that has been lyophilized.
- Conducting laboratory research: SWFI is also key in several laboratory procedures, including cell medium preparation.
- Cleaning medical devices: Surgical equipment must remain bacteria- and microorganism-free. SWFI is used to flush medical instruments to limit bacterial growth.
- Performing wound care: SWFI can also be used to clean wounds before disinfection.
- Delivering medicine: WFI can be used in inhalation therapy to deliver medicine into the lungs.
Methods for Producing WFI
Bacteriostatic Water for Injection is made the same way Sterile Water for Injection is produced, with the exception of the additive to the bacteriostatic WFI. All the production methods for WFI include water softening and carbon filtration as a means of scale control and dechlorination of the feed water source. However, each process is different depending on the method.
There are three most common systems used in the production of WFI water systems. All of these include water softening and carbon filtration as a means of scale control and dechlorination of the feed water source.
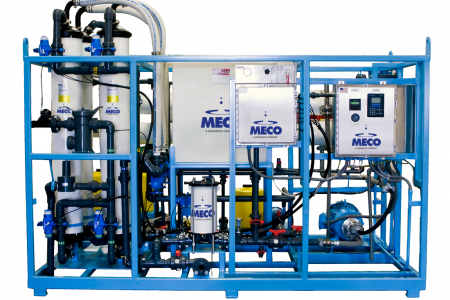
Multiple-Effect Distillation
Multiple Effect (ME) distillation remains the most commonly used method of producing WFI globally. Since energy costs in a multiple-effect system can be high, larger-capacity systems will typically have more effects to reduce the energy input to the system. Smaller capacity systems may only have three to five effects, which results in lower capital cost for the end user.
Multiple–effect distillation (utilizing higher temperatures) normally pretreats feed water at a minimum with reverse osmosis to remove dissolved ions that would otherwise promote scale or distiller corrosion. The RO unit effectively removes dissolved ions, bacteria, viruses, and suspended solids. The RO unit will normally have a minimum pretreatment system consisting of water softening, carbon filtration, and cartridge filtration. These three remove hardness, chlorine, and particulate matter, respectively.
Given the operating pressures of both RO and ME, it is common to store water in an intermediate storage tank with a feed water pump. Some pretreatment systems are subject to periodic chemical cleaning. Hot water sanitation systems can also be used, but they can increase capital and operating costs. Most ME Still–generated WFI systems store the water in a hot tank and distribute it hot to all Points of Use.
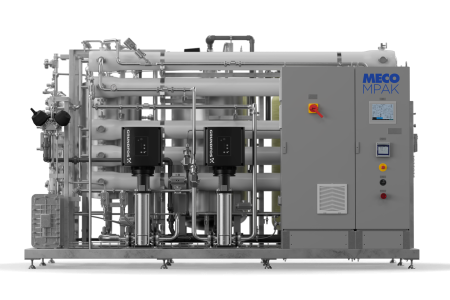
Membrane-Based Reverse Osmosis
The second method of WFI production is less common, pairing reverse osmosis with ultrafiltration technologies to achieve pharmaceutical-grade purity. The best membrane-based reverse osmosis systems have the appropriate pretreatment, deionization, and ultrafiltration functions. The RO/EDI/UF systems can be designed with ultraviolet light, double pass RO, membrane degasification, and hot water sanitization to improve the system’s functionality.
The reverse osmosis stage filters out the majority of the impurities, while the remaining dissolved ions are removed through electrodeionization. Ultrafiltration is used as a final step to remove any bioburden and endotoxin that may still be in the water.
The product water recovery rate is ~70%, considering backwashing and rinsing of the softeners and carbon vessel, and reject from the RO and EDI. Water storage is typically at ambient temperatures, similar to the WFI production temperature.
Typically, the system is hot water sanitizable for biogrowth control, although some systems are equipped for chemical cleaning/sanitization. Other generation systems are heated to self-sanitizing temperatures before feeding the storage tank and maintained at said temperatures. Ozone can also be used to continuously disinfect the WFI in the storage tank, but ozonated WFI is not sent to the Points of Use. An ultraviolet light is used to destroy it before distribution.
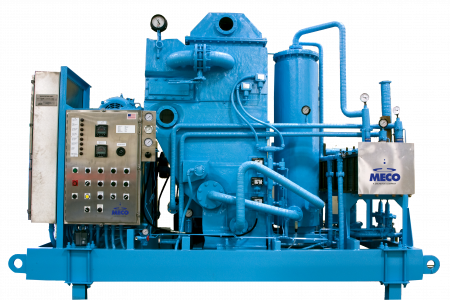
Vapor Compression Distillation

Vapor Compression (VC) distillation operates at lower temperatures than Multiple–Effect Stills, making them less susceptible to scaling and corrosion. Therefore, they can operate on a simplified pretreatment system (typically softened, dechlorinated feedwater) without requiring reverse osmosis. Vapor Compression Stills can produce water at both hot (80°C) and ambient temperatures (~10-12°C above feed water temperature). The distiller can switch between the ambient mode and hot production through opening and closing the valve around the distillate heat exchanger(s).
One of the major advantages of an ambient (cold) VC Still is that it can periodically sanitize the storage and distribution system with hot water from the distiller. It also recovers more heat and requires less plant steam than a system that produces WFI hot.
Another benefit of VC distillation is that any leak within the evaporator will present itself as high conductivity on startup, alerting users to problems early on. This is because the distilled water is processed at a higher pressure than the feed water, so any leakage progresses from the distilled water side of the heat transfer surface to the feed water side (as opposed to the other way around). In a membrane-based system, the feed water is always at a higher pressure than the product water, and a loss of integrity in any membrane system or the EDI will negatively affect the water quality.
Historically, the compressors in vapor compression systems have been criticized for a lack of reliability. However, modern advancements and diagnostics have made these compressors highly dependable, and maintenance is often accomplished in a couple of hours, if required.
(6) PES3000MSS MECO Vapor Compression Distillation units producing up to 3,000 GPH of WFI each
WFI Testing and Sampling Process
Routine sampling of TOC, conductivity, bacteria, and endotoxin levels is performed through sample valves located throughout the water system. Highly trained technicians collect samples and submit them for testing. The samples are tested against the various Pharmacopeia requirements. Caution is taken to avoid false-positive results from contamination during the collection through improper techniques, poor hygienic habits, or inadequate sterilization methods.
Partner with MECO
Water for Injection is an essential element in the biopharmaceutical manufacturing process. Many production methods can be implemented based on several factors, including capacity, existing infrastructure, risk, reliability, and energy efficiency requirements for each facility. MECO delivers Water for Injection production through highly engineered processes, averaging over 25 million gallons of pharmaceutical-grade water daily.
Our Purified Water (PW) and Sterile Water for Injection (WFI) systems serve the continual demand for use in the medical and pharmaceutical industry. The experts at MECO can help businesses navigate systems to find the best solution for their needs. MECO’s water purification specialists can implement custom processes to achieve Water for Injection production for any facility’s needs. Contact our team to learn more.